-
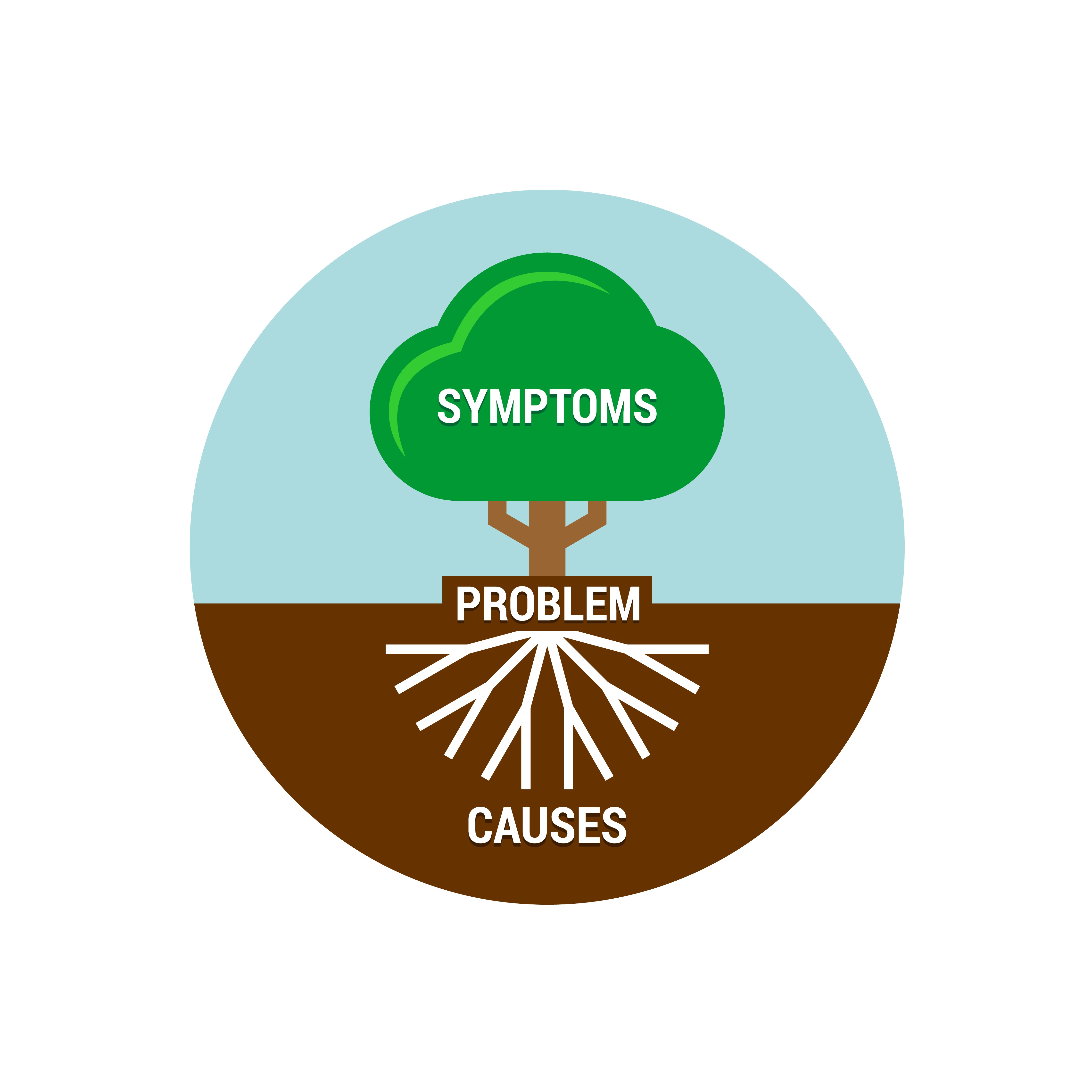
CHALLENGE
Building quality into the product, with compressing timelines, complex therapeutics, and overburdened staff.
-
SOLUTION
Collaborating with you to implement a Voluntary QA (VQA)® Culture to accelerate your time to market, reduce costs, and protect patient lives without overburdening employees.
A Scientist and an Entrepreneur at heart, learn more about Judy and her collaborative approach to quality.
WHY CARMODY QS?
Your business is unique. Your people are unique. Your products are unique. At CQS, we know that and so we work with you to provide customized solutions that translate your innovation to success and accelerate your product’s path to market. CQS is unlike many traditional consultancies. We don’t offer cookie-cutter solutions.
CQS consultants have extensive knowledge and experience in senior-level roles in operations, clinical, non-clinical, quality, and IT, and understand the challenges you are facing as we have been where you are now. As such, we provide a range of support from professional advice and expertise sharing to interim management, hands-on implementation, and training.
Awards Winning
Our Equipments
Field Expertise
Industry Expertise
What We Do
Our Main Services
Strategic Management
From Strategic Planning and Organizational Change Management to producing customized documentation for required regulatory standards.
QA Services
From creating and implementing quality management systems, including robust training programs, to building vendor selection and oversight programs based on risk.
QC Services
Offering includes ICH Q2 compliant method validation programs, ICH Q1 compliant stability programs, and GAMP compliant equipment qualification and computer software validation.
Clinical Consulting Services
We support your clinical needs from crafting comprehensive policies and procedures to qualifying your vendors. Our team offers expert guidance and professional insights, spanning from interim QA management support to hands-on implementation of robust training programs.
Training Services
Bringing 30+ years of industry experience plus a unique approach to Life Sciences training.
LATEST RESEARCH
The 4 Pillars of Our Quality Approach

Setting Expectations
People operate better when they know what is expected of them. Setting expectations and communicating with them can result in greater efficiency and productivity.

Accountability
Accountability to expectations ensures performance. When employees understand what’s expected of them, and have the proper training, they take ownership and want to succeed.

Download our new eBook today.
Driving Quality Innovation and Culture in the Pharmaceutical and Biotechnology Industries, Carmody Quality Solutions (CQS) works with pharmaceutical and biotech companies to build a proactive collaborative approach to Quality through risk-based, scientific solutions for your specific needs. CQS helps you remain compliant, while remaining sane.

Testimonial
Testimonials

Chief Executive Officer and Co-founder of a biotechnology company
Judy is a great Quality lead for any start-up life sciences company. I highly recommend Judy because she understands how companies can think about and integrate quality early on. Her approach and mindset helped make quality a core element of our business and our company culture.

Director of Quality Assurance at a Clinical-Stage Biotech Company
From my first conversation with Judy, it was clear she would make a significant impact. And she is by helping us build an outstanding and solid Quality Management System. As a partner, Judy is flexible in her approach, so our people feel part of the process, while making sure that we comply-and that people understand how it all works together and is applicable to their role.

Executive Director of Clinical Operations of a biotechnology company developing ADC technology
Dr. Carmody is knowledgeable, passionate, and personable with strong soft skills that allow her to navigate the specific quirks of any complex biotechnology organization. I highly recommend her based on her track record with us.
latest news
News & Articles
Setting a Successful Quality Strategy From Day One
“Retrofitting quality into a biotech firm is the...
Why And How To Find A Better Quality Management Tool For Your GxP-Regulated Organization
When it comes to GxP regulation, if it...
Achieving GxP Compliance For Your Spreadsheets With An Affordable, Easy-to-Use Excel Plugin
At a workshop ... the organizer gave us...
Setting a Successful Quality Strategy From Day One
“Retrofitting quality into a biotech firm is the...
Why And How To Find A Better Quality Management Tool For Your GxP-Regulated Organization
When it comes to GxP regulation, if it...
Achieving GxP Compliance For Your Spreadsheets With An Affordable, Easy-to-Use Excel Plugin
At a workshop ... the organizer gave us...
Investigating Failure For Future Success: Root Cause Analysis
At a workshop ... the organizer gave us...
A Fire Extinguisher in the Corner: “Voluntary QA” Can Bulletproof Research Practices and Data Collection
A great article in January’s Nature spells out what I...